Often the first thing on our minds when we wake up and an ever-reliable companion during long work hours, caffeine is integral to our daily lives. But what exactly is caffeine made of chemically? In essence, it’s a compound known as 1,3,7-trimethylxanthine. Let’s dive into the molecular world of this ubiquitous stimulant.
A Closer Look at Caffeine’s Chemistry
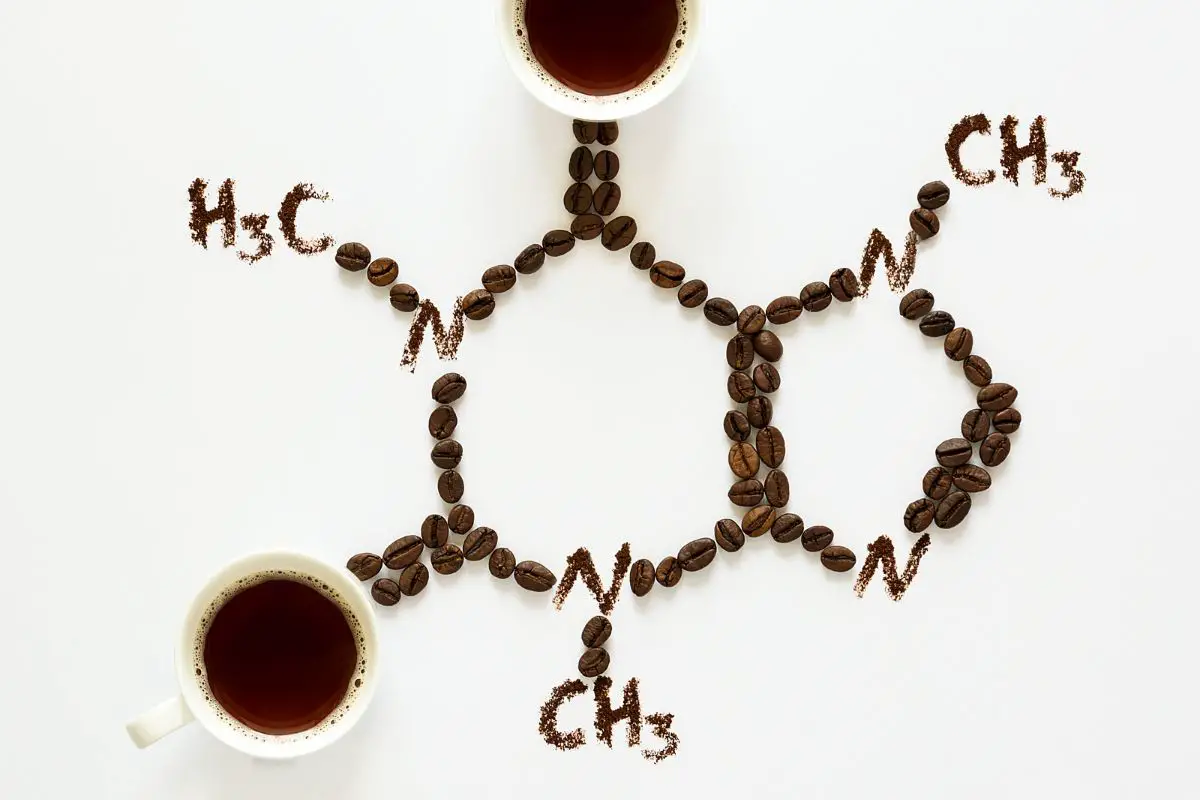
Caffeine belongs to a category of compounds known as xanthines. Its molecular formula is C8H10N4O2, which means that each caffeine molecule consists of 8 carbon atoms (C), 10 hydrogen atoms (H), 4 nitrogen atoms (N), and 2 oxygen atoms (O).
The Structural Layout
Within the caffeine molecule, these atoms are arranged to form a complex structure that consists of a fused-ring system. This includes a pyrimidinedione and an imidazole ring. It is the unique arrangement of these rings and atoms that sets caffeine apart from other similar compounds.
The Methyl Groups
Key to caffeine’s structure are the three methyl groups (-CH3). These groups are attached to the nitrogen atoms in the molecule’s structure. The position of these methyl groups differentiates caffeine from other xanthines.
This specific structure allows caffeine to interact with certain neurochemical pathways in the human body, resulting in the familiar stimulant effect that keeps sleepiness at bay.
Diving Deeper: Caffeine’s Interaction in the Body
Caffeine’s distinctive chemical structure allows it to fit perfectly into receptors in the brain designed for a neurotransmitter called adenosine. By blocking these receptors, caffeine prevents adenosine from inducing feelings of tiredness.
Enhancing Your Understanding of Caffeine
Understanding its Effects
Recognizing the chemical composition of caffeine empowers you to better understand its effects on the body. For example, knowing that caffeine is a natural antagonist of adenosine can shed light on why too much caffeine can lead to a feeling of restlessness or insomnia.
Responsible Consumption
Understanding the effects of caffeine also promotes healthier, more mindful consumption. Moderation is key; experts generally agree that up to 400 milligrams of caffeine a day (about four 8 oz. cups of coffee) is safe for most healthy adults.
The Caffeine Challenge
Now that you’re equipped with this knowledge, we invite you to observe your own reactions to caffeine. Can you notice the stimulant effects that the chemical composition of caffeine brings about? How does your daily caffeine intake align with the recommended guidelines?
Feel free to share your observations and experiences. Your personal insights will enrich our collective understanding of caffeine’s role in our daily lives.
Conclusion
Caffeine’s journey from a humble compound—1,3,7-trimethylxanthine—to a global phenomenon is truly fascinating. Its unique chemical composition, characterized by the molecular formula C8H10N4O2 and the precise arrangement of carbon, hydrogen, nitrogen, and oxygen atoms, is what makes your morning coffee or tea an effective wake-me-up beverage.
Understanding caffeine’s chemistry deepens our appreciation for that delightful cup of coffee and reminds us to enjoy it responsibly. After all, knowledge, much like a good cup of coffee, is empowering.
So, here’s to more stimulating journeys into the fascinating world of coffee and caffeine. Happy exploring!
Related article: